Many college students spend their Friday nights having a good time with their friends at the local bars. Those that may have had a little too much fun though may find getting out of bed the next morning to be a daunting and difficult task. The culprit: the hangover. We all know that alcohol can cause everything from improved dance moves to drowsiness and difficulty walking, but what does alcohol actually do inside the body that causes the hangover effects such as headaches and nausea? This question is what led me to look into the biochemistry of a hangover.
“The Hangover” is experienced differently by everyone. Some people are more prone to headaches, while others may experience more nausea. These differences are what make a hangover difficult to both define and study. Chemically however, the symptoms are rather similar including dehydration, hypoglycemia and acidosis, gastrointestinal tract issues, headaches, and sleep and circadian rhythm disruptions.
For example, alcohol is a diuretic and for every 50 g of ethanol in 120 mL of liquid (about 3 shots), the body eliminates between 600 and 1000 mL of water. Additionally, ethanol decreases antidiuretic hormone vasopressin secretion, resulting in even more urine excretion. Any additional sweating and vomiting will also dehydrate the body even more. A variety of hormones that are secreted under the influence of alcohol can also disrupt circadian rhythms and sleep patterns even though alcohol can act as a narcotic.
The mood swings and weakness during a hangover can be blamed on a decrease NAD+:NADH ratio in the body, leading to lactic acid build up and low glucose production. A drop in blood glucose concentration leads to the weakness and mood swings associated with a hangover. Even low alcohol content drinks will induce stomach acid production, resulting in nausea the next morning. Finally, the splitting headaches that 66% of people experience during hangovers are a direct result of alcohol’s ability to expand blood vessels and influence the production of neurotransmitters.
In addition to ethanol, Figure 1 shows the amounts of common compounds in certain types of alcohol. Brandy, with its high concentration of methanol, has been proven to cause the worst hangovers. How does science suggest curing a hangover? Lots of water and “compassion and words of comfort from loved ones”.
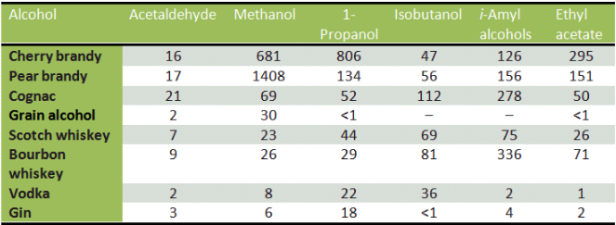
Figure 1: This figure shows the amounts of different compounds in common types of alcohol. High concentrations of methanol can lead to methanol poisoning and can even lead to the production of formaldehyde inside the body. Methanol will only be broken down after complete metabolism of ethanol, so drinks containing both ethanol and methanol can have severe effects on the body. Hangovers involving high methanol content are sometimes treated by adding ethanol to the body in order to gain time for dialysis by blocking methanol oxidation.
- Megan K.
References (same source for text and Figure 1)
- Roth, Klaus. “Chemistry of a Hangover – Alcohol and Its Consequences Part 3.”org. Chemie in Unserer Zeit/Wiley-VCH, 6 July 2011. Web. 30 June 2016.
- URL: http://www.chemistryviews.org/details/ezine/1080019/Chemistry_of_a_Hangover__Alcohol_and_its_Consequences_Part_3.html

That was written very clearly, and was enjoyable to read. Do you know why some people tend to have more headaches while some people tend to have more nausea?
LikeLike
Really interesting read. I thought the portion about methanol was most interesting. It makes me wonder why high methanol content makes headaches so bad the next day. Is it just because it can linger in your body for longer periods of time after ethanol is metabolized, or is there a more complex mechanism?
LikeLike